Imagine inhaling a gas that smells like rotten eggs. That’s the pungent scent of sulfur dioxide, a compound naturally released from volcanic eruptions or during the burning of fossil fuels. But beyond its unpleasant odor, sulfur dioxide plays a crucial role in our atmosphere, affecting climate and even impacting human health. Understanding whether SO2 is polar or not is paramount to grasp its environmental implications, so let’s dive into the fascinating world of sulfur dioxide.
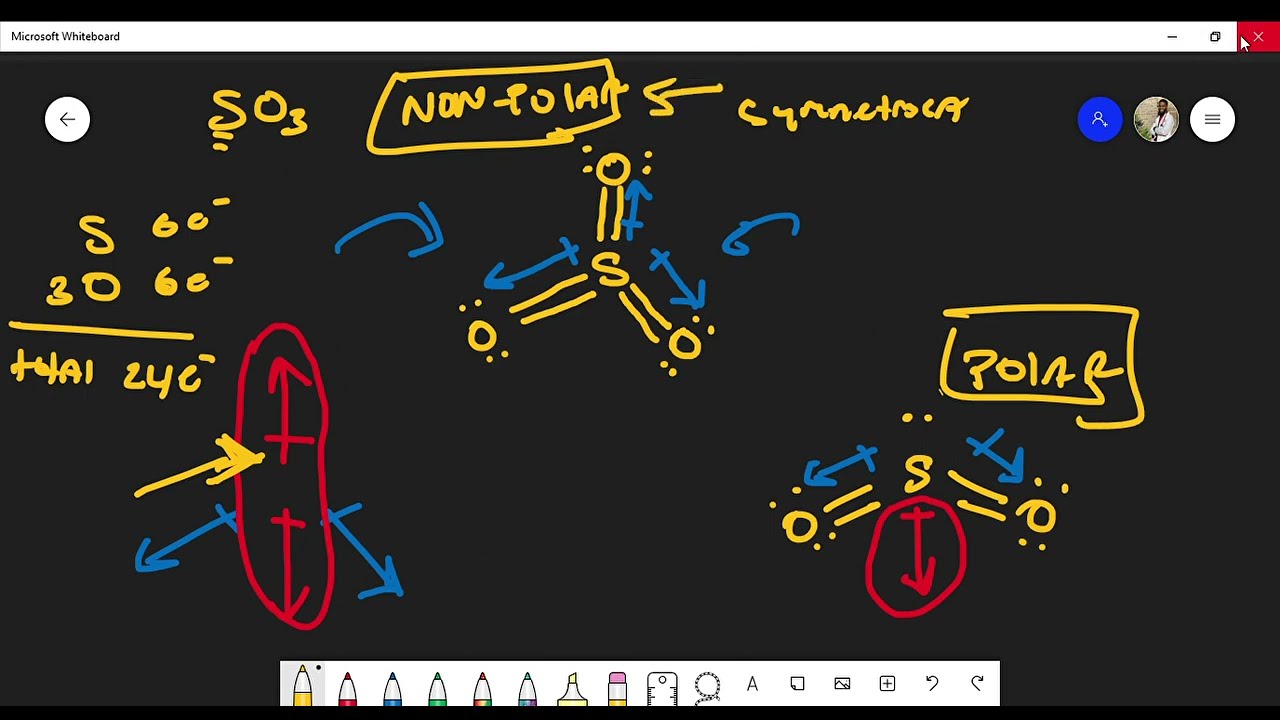
Image: www.youtube.com
The concept of polarity in chemistry refers to the uneven distribution of electrical charges within a molecule. A molecule is considered polar when one end carries a slightly positive charge (δ+) and the other end carries a slightly negative charge (δ-). This uneven distribution arises due to differences in the electronegativity of the atoms that make up the molecule. In the case of sulfur dioxide (SO2), the question of polarity becomes an intriguing puzzle – is it a polar molecule or does it lack this distinct charge separation?
Unveiling the Molecular Structure of SO2
To understand whether SO2 is polar, we need to delve into its molecular structure. Sulfur dioxide consists of a central sulfur atom bonded to two oxygen atoms. Both sulfur and oxygen have six valence electrons, meaning they each have six electrons in their outermost shell.
Bent Molecular Geometry
To achieve stability, the sulfur atom in SO2 forms two double bonds with the oxygen atoms. A double bond signifies a shared pair of electrons, giving each oxygen atom a full octet. However, the sulfur atom, despite sharing its electrons, still has a lone pair of electrons remaining. This lone pair, along with the two double bonds, creates a bent or V-shaped molecular geometry around the sulfur atom, like a boomerang.
The Role of Electronegativity
The oxygen atoms are more electronegative than the sulfur atom. Electronegativity is the ability of an atom to attract electrons towards itself within a bond. Since oxygen is more electronegative, it attracts the shared electrons in the SO2 bonds towards itself, creating a partial negative charge (δ-) on the oxygen atoms and a partial positive charge (δ+) on the sulfur atom.

Image: www.youtube.com
The Verdict: SO2 is a Polar Molecule
The combination of the bent molecular geometry and the difference in electronegativity between sulfur and oxygen results in a net dipole moment for SO2. A dipole moment is a measure of the separation of positive and negative charges within a molecule. Since SO2 has a non-zero dipole moment, it is considered a polar molecule.
Implications of Polarity
This polarity has significant implications for how SO2 interacts with its environment and behaves in different chemical reactions. We’ll explore some of these implications in the next section.
The Environmental Impact of SO2
Sulfur dioxide is a major air pollutant released into the atmosphere through natural sources like volcanic eruptions and human activities like burning fossil fuels and industrial processes. The polarity of SO2 contributes to its environmental impact in several ways.
Acid Rain Formation
One of the most critical environmental impacts of SO2 is its role in acid rain formation. When SO2 dissolves in water droplets in the atmosphere, it reacts with oxygen to form sulfuric acid (H2SO4). Sulfuric acid is a strong acid that eventually falls back to Earth as acid rain.
Respiratory Issues
Sulfur dioxide is a respiratory irritant that can cause coughing, wheezing, and shortness of breath in humans. This is due to its ability to dissolve in the moist lining of the respiratory system, where it reacts with water to form sulfuric acid, damaging lung tissue.
Global Climate Change and SO2
Sulfur dioxide also plays a complex role in global climate change. While it can contribute to acid rain, which negatively affects ecosystems, it can also have a cooling effect on the planet.
Cooling Effect
Sulfur dioxide released into the atmosphere can form sulfate aerosols which reflect sunlight back into space, thus reducing the amount of solar radiation reaching the Earth’s surface. This leads to a cooling effect on the climate.
Volcanic Eruptions and Climate
A noteworthy example is the eruption of Mount Pinatubo in 1991, which released a massive amount of SO2 into the atmosphere. The sulfate aerosols formed from this eruption led to a temporary cooling of the Earth’s surface.
Monitoring and Controlling SO2 Emissions
Understanding the impact of SO2 on the environment and human health is crucial for developing effective strategies to monitor and control emissions. Numerous technologies have been developed to reduce SO2 emissions from industrial sources, such as:
Flue Gas Desulfurization
This technology involves scrubbing sulfur dioxide from flue gases produced during industrial processes using a variety of techniques, including wet scrubbing and dry scrubbing. In wet scrubbing, flue gas is passed through a solution that dissolves SO2 and forms a disposable byproduct. In dry scrubbing, SO2 reacts with a dry absorbent, forming a solid byproduct that can be disposed of or used to make gypsum.
Catalyst-Based Technologies
These technologies use catalysts to convert SO2 into less harmful compounds or to remove it from the flue gas. For example, selective catalytic reduction (SCR) technology uses a catalyst to convert SO2 to sulfur trioxide (SO3) and then to sulfuric acid. This sulfuric acid can be neutralized with an alkali solution to form a harmless salt.
Is So2 Polar
Conclusion
The story of SO2 is a complex interplay of chemistry and its environmental implications. We now understand that SO2 is a polar molecule due to its bent molecular geometry and the difference in electronegativities between sulfur and oxygen. This polarity leads to SO2’s significant impacts on the environment, including the formation of acid rain and its role in global climate change. Through effective monitoring and the use of innovative technologies, we can manage the release of SO2 and reduce its harmful effects. This knowledge enables us to manage this critical compound and work towards a healthier planet.



/GettyImages-173599369-58ad68f83df78c345b829dfc.jpg?w=740&resize=740,414&ssl=1)


