Have you ever wondered what makes up the atoms that form the entire universe? The world around us is a tapestry woven by the interactions of tiny, invisible particles – atoms. But what lies at the heart of these fundamental building blocks? The answer lies in the intricate dance of electrons, protons, and neutrons. In this exploration, we’ll delve into the fascinating world of atomic structure and answer the question: does potassium have more electrons than neon?
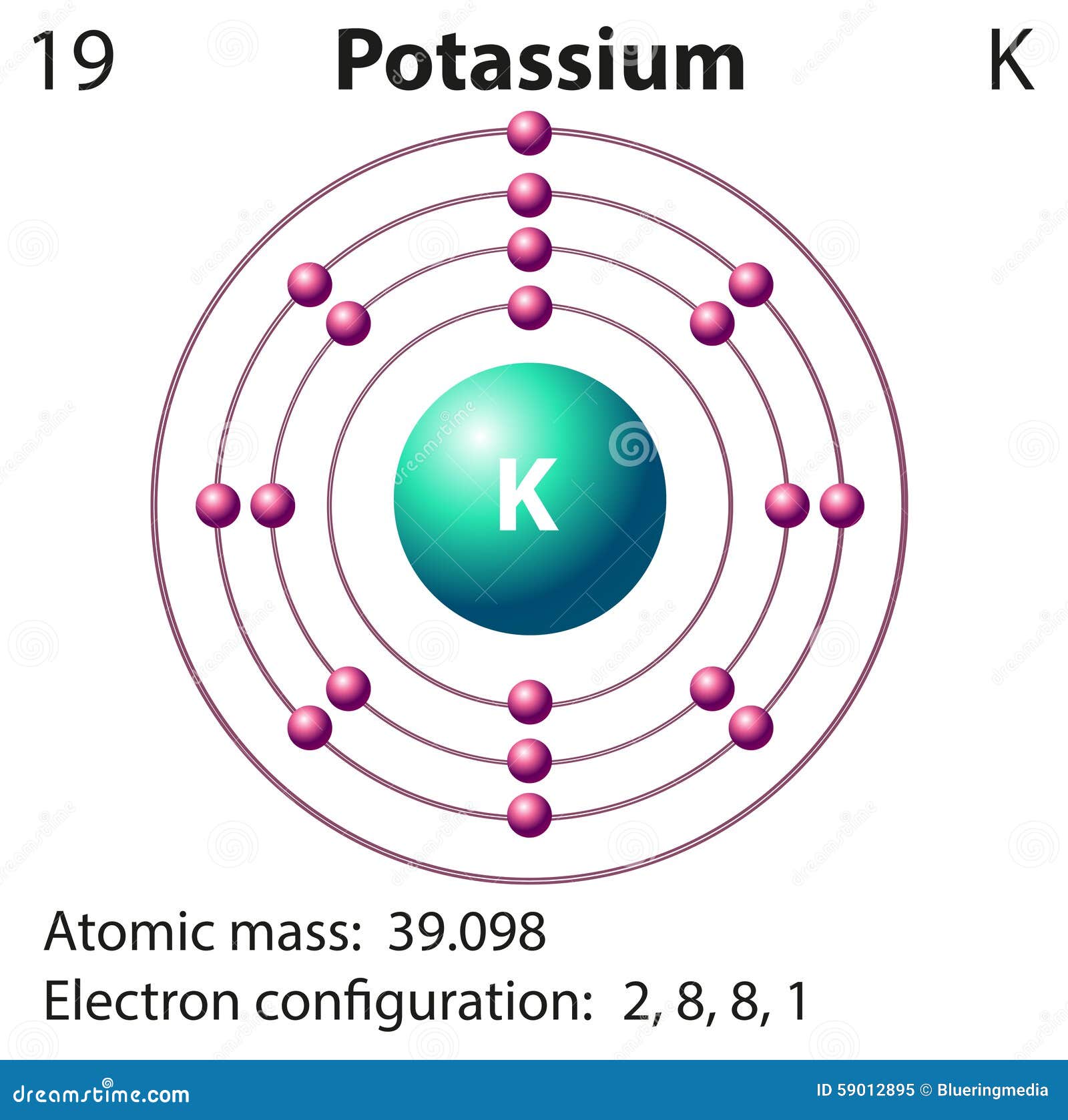
Image: www.dreamstime.com
Understanding the number of electrons in an atom is key to comprehending how elements behave and interact with one another. It’s an essential concept in chemistry, physics, and even biology, as it helps us grasp the foundations of chemical reactions, the properties of materials, and even the complex processes that drive life itself. So, let’s embark on a journey into the fascinating realm of atomic particles and unravel the secrets behind potassium and neon.
Atomic Structure: The Building Blocks of Matter
The Electron’s Role in the Atomic Orchestra
At the center of every atom resides the nucleus, a dense core composed of protons and neutrons. These particles carry a positive charge (protons) or are neutral (neutrons). Orbiting around this nucleus, like planets around a sun, are negatively charged electrons. The number of protons in an atom determines its atomic number, defining its identity as a specific element. For example, all potassium atoms have 19 protons, while all neon atoms contain 10 protons. This means potassium and neon are fundamentally distinct elements.
Electron Shells: Organized Chaos in the Atom
Electrons aren’t just randomly zipping around the nucleus. They occupy specific energy levels, visualized as shells surrounding the nucleus, like layers of an onion. Each shell can accommodate a maximum number of electrons. The first shell, closest to the nucleus, can hold up to two electrons. The second shell can hold up to eight, and so on. The arrangement of electrons in these shells dictates an element’s chemical behavior.
Image: socratic.org
Unveiling the Electron Count: Potassium vs. Neon
Potassium: An Energetic Player
Potassium, a silvery-white metal, finds itself in the first column of the periodic table, alongside other highly reactive metals like sodium and lithium. Its atomic number is 19, meaning it has 19 protons in its nucleus. To maintain a neutral charge, potassium also has 19 electrons orbiting around the nucleus. These electrons are distributed in four shells: two in the first shell, eight in the second, eight in the third, and just one in the outermost fourth shell.
Neon: The Noble Gas with a Full Shell
Neon, a colorless, odorless gas, sits in Group 18 of the periodic table, known as the noble gases. Noble gases are renowned for their inertness, meaning they resist forming chemical bonds with other elements. Neon, with an atomic number of 10, has 10 protons and, accordingly, 10 electrons. These electrons are neatly arranged in two shells: two in the first and eight in the second. With a full outer shell, neon is incredibly stable and unreactive.
The Answer: Potassium Has More Electrons Than Neon
Comparing the electron configurations of potassium and neon, it becomes evident that potassium has 19 electrons compared to neon’s 10. Thus, potassium does have more electrons than neon. This difference in electron count is a direct consequence of their varying atomic numbers and reflects their distinct chemical properties.
Implications of Electron Count: Reactivity and Bonds
Potassium’s Eagerness to React
The lone electron in potassium’s outermost shell makes it highly reactive. It’s desperate to find another electron to complete its outer shell and achieve stability. This eagerness drives potassium to readily participate in chemical reactions, losing its outermost electron to form positive ions (K+) and forming ionic bonds with other elements.
Neon’s Unwillingness to Participate
Neon, on the other hand, has a full outer shell. It’s already content and stable, lacking the urge to gain or lose electrons. This full shell makes neon incredibly unreactive, explaining its existence as an inert gas in the atmosphere. The principle of a full outer shell being a stable configuration is a fundamental concept in chemistry and helps explain the bonding behavior of all elements.
Beyond the Basics: Electrons and the World Around Us
Electrons in Everyday Life
The electron count of elements has far-reaching consequences in our daily lives. It helps us understand why metals are good conductors of electricity (electrons can move freely), why salt dissolves in water (ionic bonds between sodium and chlorine), why plastics are insulators (electrons are tightly bound), and why batteries store energy (electrons flow from one electrode to another).
Electrons in Technology
Electrons are the lifeblood of modern technology. They power our computing devices, smartphones, and even the lights in our homes. The flow of electrons through semiconductors enables transistors, the foundation of modern electronics. The understanding of electron behavior has driven the development of lasers, LEDs, and other revolutionary technologies that shape our world.
Does Potassium Have More Electrons Than Neon
Conclusion: A Deeper Appreciation for the Atomic Realm
By unraveling the secrets behind atomic structure and electron count, we gain a deeper appreciation for the world around us. Understanding the differences between elements like potassium and neon allows us to comprehend their diverse chemical properties and their roles in various reactions and applications. From the fundamental building blocks of matter to the cutting-edge technologies that define our era, electrons play a vital role in shaping our universe. So, next time you glance at a piece of potassium or see a neon sign light up, remember the fascinating world of atomic particles that makes it all possible.



/GettyImages-173599369-58ad68f83df78c345b829dfc.jpg?w=740&resize=740,414&ssl=1)


